
By Warren R. Heymann, MD
January 13, 2021
Vol. 3, No. 2

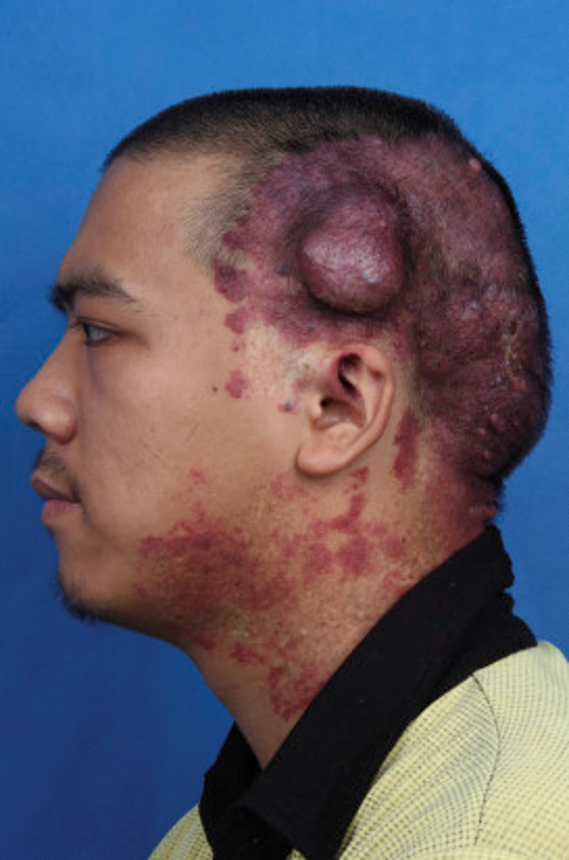
Port wine stains (PWS, aka nevus flammeus, nevus simplex, salmon patch) are cutaneous capillary malformations that are characteristically congenital (CPWS) but may be acquired (APWS). APWS was first described in a German publication by Fegeler in 1949, hence the term Fegeler syndrome. To date, fewer than 100 cases of APWS have been described. (1) CPWS affect 0.3-1% of newborn infants; rare APWS mostly develop later in life. (2)
CPWS have no sex predilection and they usually appear sporadically. Typically, they are initially pink and flat, but with time darken, and may thicken with developing vascular nodules, as noted in two-thirds of patients by age 50. Approximately 90% are distributed on the head, although all sites may be involved. (3) CPWS may be associated with several syndromes, most notably Sturge-Weber, Klippel-Trenaunay, and Proteus, among others. (1) Histologically, CPWS and APWS are identical. A normal number of ectatic vessels are observed, lined by unremarkable endothelial cells without any proliferation. Progressive fibrosis develops around the vessels at later stages. Diminished nerve density has been demonstrated within the lesions. (4) Treatment of PWS focuses on the pulsed dye laser. (2)
Recently, evidence has implicated somatic genetic mutations (GNAQ, PI3K), MAPK and PI3K aberrant activations in the pathogenesis of PWS. According to Seremet et al: “Current data support that: (1) PWS is a multifactorial malformation involving the entire physiological structure of human skin; (2) PWS should be pathoanatomically re-defined as ‘a malformation resulting from differentiation-impaired endothelial cells with a progressive dilatation of immature venule-like vasculatures’; (3) dysregulation of vascular MAPK and/or PI3K signaling during human embryonic development plays a part in the pathogenesis and progression of PWS/SWS; and (4) sporadic low frequency somatic mutations, such as GNAQ, PI3K, work as team players but not as a lone wolf, contributing to the development of vascular phenotypes.” (3)
One of the prevailing hypotheses of the etiology of PWS suggests that the diminished nerve function within the lesions causes a lack of [sympathetic] neuromodulation of vascular tone, leading to progressive vascular ectasia. (5) This would explain why trauma is the most likely cause of APWS; however, in a series of 59 reviewed cases, only 17 (29%) were attributed to traumatic injury, often sports-related. (6) Other forms of injury could include car accidents (7) or sunburn. (4) Medications have been associated with APWS, including isotretinoin (presuming increased skin fragility), oral contraceptives, simvastatin, and metformin (the latter two are believed to promote angiogenesis by upregulation of VEGF). (2)
Although I am confident that I have seen rare cases of APWS, the most memorable being a lesion on the scalp in a young man following head trauma, I have also been fooled by cases that mimic morphea (8), angiosarcoma, and cutaneous B-cell lymphoma. Because there are so few APWS in the literature, unless there are compelling reasons not to (especially in adults), biopsies should be routinely performed.
My primary motivation in writing this commentary was my (mis)perception that APWS are only seen in adults. Stephens et al presented a case series of 6 children (3 to 11 years old) with APWS without any history of trauma or known inciting factors. Genetic studies were performed in two patients, neither of which demonstrated any of the mutations associated with PWS. One child had a deletion of chromosome 16p11. (9) A PubMed search of the 16p11.2 microdeletion syndrome did not reveal any cases associated with PWS. Clearly, more research is required to elucidate the pathomechanism(s) of APWS in children.
Samuel Johnson stated: “This is one of the disadvantages of wine, it makes a man mistake words for thoughts.” When it comes to comprehending acquired port wine stains much thinking remains ahead.
Point to Remember: APWS are rare but affect all age groups. While trauma is the most likely culprit in these cases, there are potentially other predisposing factors, which may (or may not) be based on genetic predisposition.
Our Expert’s Viewpoint
Marissa Perman, MD
Associate Professor of Dermatology
Associate Professor of Clinical Pediatrics
Perelman School of Medicine at the University of Pennsylvania
We learn fairly early in dermatology residency that capillary malformations (CM), also referred to as port wine stains (PWS), are static vascular malformations that are present at birth which often helps to distinguish them in the early phase from other neonatal vascular anomalies such as hemangioma. However, some of the newest studies on vascular malformations defy this classic teaching. For one, we know that capillary malformations present at birth are often progressive over time, in many cases developing hypertrophy and bleeding papules. We also learn in training about a subset of PWS, referred to as acquired PWS (APWS), that are traditionally thought to be caused by trauma in adults. However, like so many diseases in medicine, this traditional teaching does not fit the history every time. In pediatric dermatology clinic, we have seen several children from toddlers to teenagers whose parents confidently state (and back it up with photographs) that a PWS appeared sometime after birth without any known history of trauma and meet the description of an APWS. We are likely just beginning to uncover how these mutations cause PWS. Whether classic PWS presenting at birth versus APWS in children and adults are caused by the same mutations or have a totally different biology remains to be seen, and I look forward to the journey elucidating those pathways in the coming years.
- Hibler J, Ojung O, Kaffenberger BH. Acquired port-wine stain (Fegeler syndrome): A case report and review of the literature. J Am Osteopath Coll Dermatol 2016; 35: 15-16.
- Bansal S, Garg VK, Wadhwa B, Khurana N. Acquired port wine stain in an adult male: First reported case from India with review of the literature. Indian J Dermatol 2015; 60: 104.
- Nguyen V, Hochman M, Mihm, MC, Jr. Nelson JS, Tan W. The pathogenesis of port wine stain and Sturge Weber syndrome: Complex interactions between genetic alterations and aberrant MAPK and PI3K activation. Int J Mol Sci 2019; 20: 2243.
- Seremet S, Benar EB, Afsar FS, Calli A, Ulusarac O. A case of acquired port wine stain: An association with repeated sunburn? Int J Dermatol 2016; 55: e544-546.
- Yang SY, Lan CC. Acquired port-wine stain with local heat. Acta Derm Venereol 2016; 96: 274-275.
- Adams BB, Lucky AW. Acquired post-wine stains and antecedent trauma: Case report and review of the literature. Arch Dermatol 2000; 136: 897-899.
- Nussbaumer-Ochsner Y, Spinas G. Dermatological sequela of a car accident: Acquired port-wine stain (Fegeler syndrome). BMJ Case Rep 2015; doi: 10.1136/bcr-2015-210860.
- Bassi A, Piccinin P, Fillippeschi, Oranges T, et al. Inflammatory morphea presenting as hemifacial acquired port-wine stain. Arch Dis Child 2019; 104: 296.
- Stephens MR, Putterman BS, Yan AC, Castelo-Soccio L, Perman M. Acquired port-wine stains in six pediatric patients. Pediatr Dermatol 2020; 37: 93-97.

